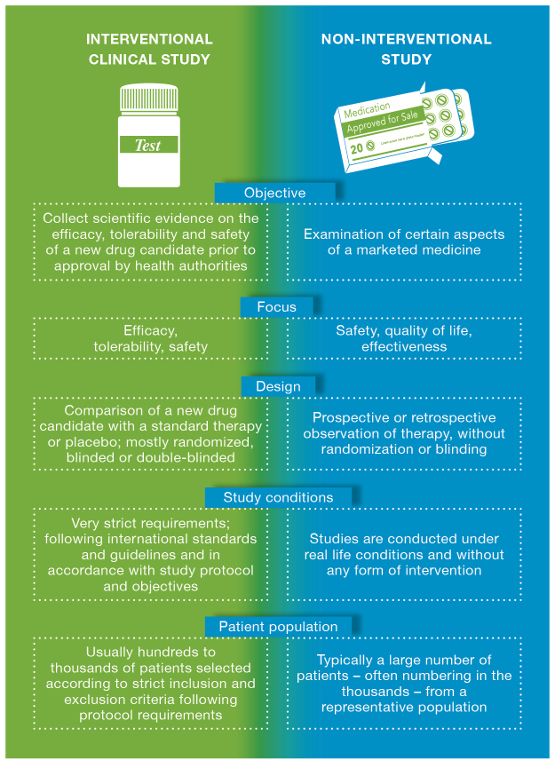
Interventional vs. Non-Interventional
Non-Interventional Study = Medicinal product is prescribed in the usual manner (Example: Acetaminophen only) Interventional Study = Participants are assigned to receive one or more interventions so that researchers can evaluate the effects of the interventions. (Study medication (Acetaminophen ) with Cantaloupe juice and Aspirin)