FAERS Public Dashboard
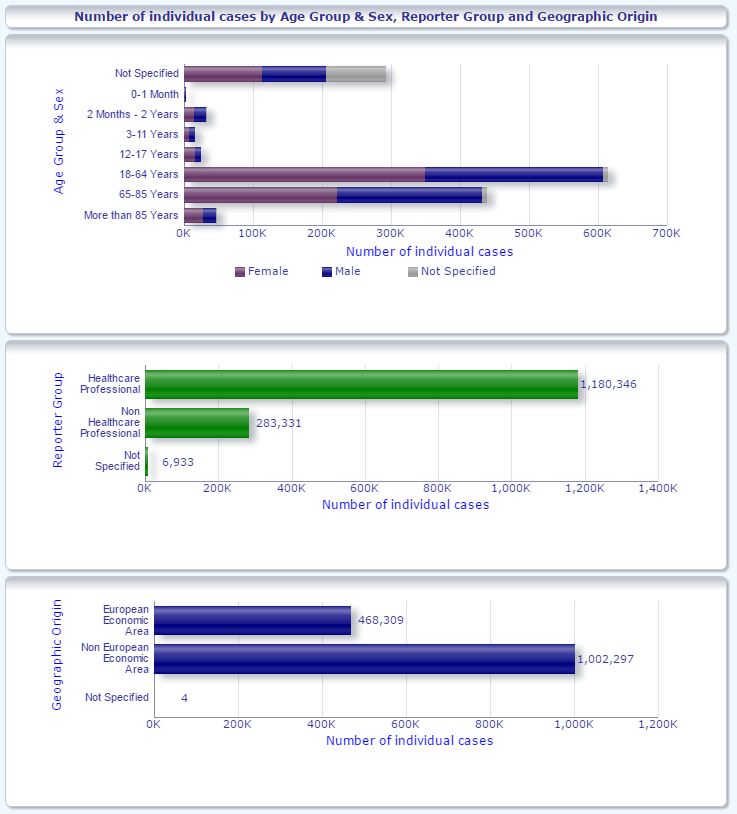
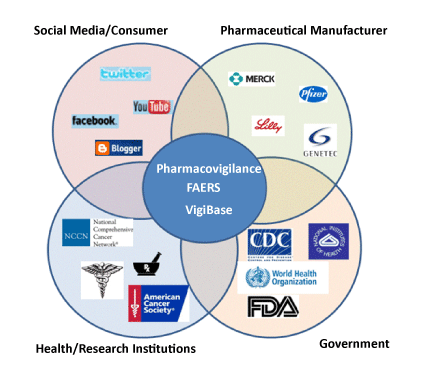
FDA’s Adverse Event Reporting System (FAERS) Public Website https://fis.fda.gov/hub/ offers dashboard by Product Report Statistics Demographic Reaction Cases Listings For Vaccines, VAERS click here The Vaccine Adverse Event Reporting System (VAERS) About (cdc.gov)