What is FAERS?
FDA Adverse Event Reporting System (FAERS)
FAERS is a database that contains information on adverse event and medication error reports submitted to FDA. It contains over 9 million reports of adverse events from 1969 to the present.
How did FDA collected this data?
Reporting of adverse events and medication errors by healthcare professionals and consumers is voluntary in the United States. FDA receives some adverse event and medication error reports directly from healthcare professionals and consumers.
How many reports (aka Cases) received by FDA from 2006 to 2015?

| FAERS YEAR | Expedited | DIRECT | Non Expedited Entered | Total Entered | Non Expedited Received | Total Received |
|---|---|---|---|---|---|---|
| 2006 | 219,217 | 20,979 | 95,555 | 335,751 | 230,065 | 470,261 |
| 2007 | 229,982 | 23,032 | 110,405 | 363,419 | 228,206 | 481,220 |
| 2008 | 274,281 | 32,896 | 132,690 | 439,867 | 218,205 | 525,382 |
| 2009 | 330,383 | 34,165 | 126,177 | 490,725 | 216,264 | 580,812 |
| 2010 | 409,547 | 28,946 | 234,646 | 673,169 | 320,341 | 758,834 |
| 2011 | 499,155 | 28,045 | 255,275 | 782,475 | 346,745 | 873,945 |
| 2012 | 577,852 | 29,026 | 326,637 | 933,515 | 475,993 | 1,082,871 |
| 2013 | 635,206 | 28,386 | 411,622 | 1,075,214 | 506,512 |
1.170,104
|
| 2014 | 746,584 | 34,246 | 423,855 | 1,204,685 | 508,303 | 1,289,133 |
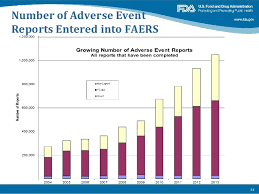
Number of Adverse Event Reports Entered into FAERS