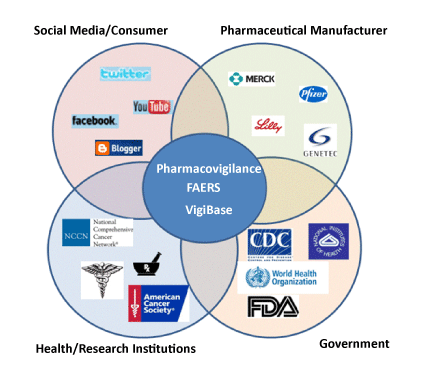
Individual Case Safety Reports (ICSRs)
Individual Case Safety Reports (ICSRs) Every country has representative who reports Medicines’ side effects to World Health Organizations (WHO). Each side effect related “a drug” (aka Primary Suspect Drug) must be reported to WHO. Valid ISCR: A Case created in the Safety system that includes the following four (4) minimum data elements: 1. Product…
Read more